Investigations into the decomposition of aminoacyl-substituted monosaccharide scaffolds from a drug discovery library†
Abstract
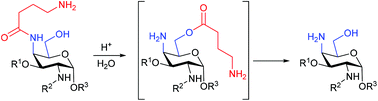
This study investigated the unexpected decomposition and associated intermediates of compound 1, a specific member of a drug discovery library based on a monosaccharide scaffold. LC/MS and NMR spectroscopic analyses indicated that, under acidic conditions, 1 can be converted into the 4-aminogalactoside 2, due to cleavage of the 4-aminobutanoyl side chain. The reaction occurs most likely through an initial intramolecular amino–amide interaction, followed by an N- to O-acyl transfer of the side chain from C-4 to the C-6 position to form an ester intermediate (5), detectable by NMR, and subsequent hydrolysis. Similar decomposition reactions could be induced in selected compounds with similar structures, containing a free hydroxyl group at C-6 and a 4-aminobutanoyl side chain at C-4 of an aminogalactoside. Furthermore, three model compounds were synthesized without a C-6 hydroxyl group and with different length aminoalkanoyl side chains at the C-4 position. The model compounds all decomposed under acidic conditions, but at different rates and much slower when compared with compound 1, suggesting that both the C-6 hydroxyl group and the length of the side chain have an influence on stability.

 Please wait while we load your content...
Please wait while we load your content...