Highly enantioselective synthesis of naphthoquinones and pyranonaphthoquinones catalyzed by bifunctional chiral bis-squaramides†
Abstract
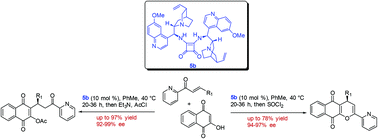
A variety of enantioenriched naphthoquinones have been synthesized in high yields and excellent enantioselectivities (up to >99% ee) using a bifunctional chiral bis-squaramide catalyzed conjugate addition of 2-hydroxy-1,4-naphthoquinone to 2-enoylpyridines. Some of the Michael products have been successfully converted into various enantioenriched pyranonaphthoquinone derivatives. The protocol is further extended to the synthesis of various 4-hydroxycoumarin derivatives under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...