N-heterocyclic carbene–palladium(ii)-1-methylimidazole complex-catalyzed Suzuki–Miyaura coupling of benzyl sulfonates with arylboronic acids†
Abstract
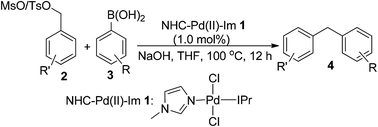
The first example of palladium-catalyzed Suzuki–Miyaura coupling between benzyl sulfonates and arylboronic acids was reported in this paper. In the presence of a well-defined, air-stable and easily available NHC–Pd(II)–Im complex, all reactions worked well to give the desired products in good to almost quantitative yields under the optimal conditions. Electron-rich, -neutral, -poor and sterically-hindered substituents on both substrates are tolerated in such transformation, providing a convenient, efficient and alternative method for the synthesis of diarylmethanes.

 Please wait while we load your content...
Please wait while we load your content...