Exploring the relationship between the conformation and pKa: can a pKa value be used to determine the conformational equilibrium?†
Abstract
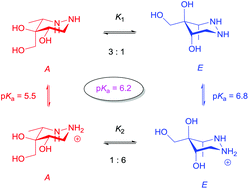
Four substituted cis and trans-4,5-dihydroxyhexahydropyridazines that were expected to undergo pH induced conformational switching were synthesized and carefully investigated by NMR analyses and calculations. For two of the compounds a large difference in pKa existed between the two possible chair conformers and for one compound this resulted in conformational switching as a result of pH change. For the first time it is shown that the pKa directly reflects the conformational equilibrium of conformers.


 Please wait while we load your content...
Please wait while we load your content...