Copper-catalyzed O-arylation of N-protected 1,2-aminoalcohols using functionalized trivalent organobismuth reagents†
Abstract
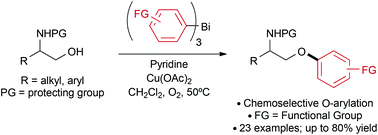
The O-arylation of 1,2-aminoalcohols using functionalized triarylbismuth reagents is reported. The reaction can be performed using substoichiometric amounts of copper acetate and operates under mild conditions. Good functional group tolerance is observed, giving access to a range of β-aryloxyamines. The effect provided by the amino group in the arylation reaction is investigated.

 Please wait while we load your content...
Please wait while we load your content...