The effect of leaving radical on the formation of tetrahydroselenophene by SHi ring closure: an experimental and computational study†
Abstract
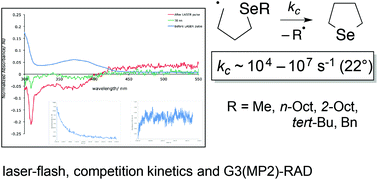
Competition kinetic studies augmented with laser-flash photolysis and high-level computational techniques [G3(MP2)-RAD], with [COSMO-RS, SMD] and without solvent correction, provide kinetic parameters for the ring closures of a series of 4-(alkylseleno)butyl radicals 1. At 22 °C rate constants (kc) that lie between 104–107 s−1 were determined experimentally and correlate with expectations based on leaving group ability. Activation energies (Eact) were determined to lie between 10.6 (R = Ph2CH) and 28.0 (R = n-Bu) kJ mol−1, while log(A/s−1) values were generally between 9 and 10 in benzene. Computationally determined rate constants were in good-to-excellent agreement with those determined experimentally, with the COSMO-RS solvation model providing values that more closely resemble those from experiment than SMD.

 Please wait while we load your content...
Please wait while we load your content...