Styryl ether formation from benzyl alcohols under transition-metal-free basic DMSO conditions†
Abstract
A phenol-catalyzed aerobic oxidative styryl ether formation method was developed with benzyl alcohol under basic DMSO. Styryl ether was obtained after 12 hours of heating at 60–80 °C where DMSO was involved in the reaction as the extra carbon source. Control experiments indicated that both phenol and DMSO are crucial for the success of the reaction. A variety of styryl ethers were prepared smoothly from benzyl alcohols in good to excellent yields in an environmentally friendly way.
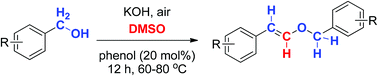

 Please wait while we load your content...
Please wait while we load your content...