Cyclopeptides containing the DEKS motif as conformationally restricted collagen telopeptide analogues: synthesis and conformational analysis†
Abstract
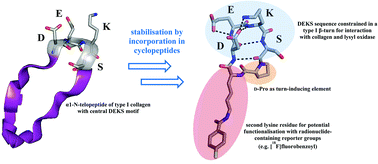
The collagen telopeptides play an important role for lysyl oxidase-mediated crosslinking, a process which is deregulated during tumour progression. The DEKS motif which is located within the N-terminal telopeptide of the α1 chain of type I collagen has been suggested to adopt a βI-turn conformation upon docking to its triple-helical receptor domain, which seems to be critical for lysyl oxidase-catalysed deamination and subsequent crosslinking by Schiff-base formation. Herein, the design and synthesis of cyclic peptides which constrain the DEKS sequence in a β-turn conformation will be described. Lysine-side chain attachment to 2-chlorotrityl chloride-modified polystyrene resin followed by microwave-assisted solid-phase peptide synthesis and on-resin cyclisation allowed for an efficient access to head-to-tail cyclised DEKS-derived cyclic penta- and hexapeptides. An Nε-(4-fluorobenzoyl)lysine residue was included in the cyclopeptides to allow their potential radiolabelling with fluorine-18 for PET imaging of lysyl oxidase. Conformational analysis by 1H NMR and chiroptical (electronic and vibrational CD) spectroscopy together with MD simulations demonstrated that the concomitant incorporation of a D-proline and an additional lysine for potential radiolabel attachment accounts for a reliable induction of the desired βI-turn structure in the DEKS motif in both DMSO and water as solvents. The stabilised conformation of the cyclohexapeptide is further reflected by its resistance to trypsin-mediated degradation. In addition, the deaminated analogue containing allysine in place of lysine has been synthesised via the corresponding ε-hydroxynorleucine containing cyclohexapeptide. Both ε-hydroxynorleucine and allysine containing cyclic hexapeptides have been subjected to conformational analysis in the same manner as the lysine-based parent structure. Thus, both a conformationally restricted lysyl oxidase substrate and product have been synthetically accessed, which will enable their potential use for molecular imaging of these important enzymes.

 Please wait while we load your content...
Please wait while we load your content...