Fluoride binding in water with the use of micellar nanodevices based on salophen complexes†
Abstract
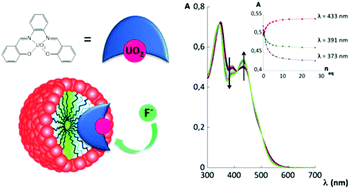
The use of micelles to transpose lipophilic receptors, such as uranyl-salophen complexes, into an aqueous environment is a valuable and versatile tool. Receptor 1 incorporated into CTABr micelles forms a supramolecular system that exhibits excellent binding properties towards fluoride in water, despite the competition of the aqueous medium. To fully evaluate the potential of micellar nanodevices, we extended our previous study to other types of surfactants and to a uranyl-salophen receptor with a more extended aromatic surface. Paramagnetic relaxation enhancement experiments were used to obtain information on the location of the two receptors within the micelles and complementary information was obtained from dynamic light scattering experiments. With these data it is possible to account for the key factors necessary to obtain an efficient supramolecular device for anion binding in water.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...