Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenative-coupling of alkynes and tetrahydroisoquinolines†
Abstract
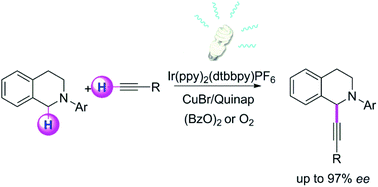
A highly efficient catalytic asymmetric alkynylation of prochiral CH2 groups in tetrahydroisoquinoline was developed using copper catalyzed cross-dehydrogenative-coupling of sp3 and sp C–H bonds with the assistance of a photocatalyst and visible light.

 Please wait while we load your content...
Please wait while we load your content...