Microwave-assisted preparation of 4-amino-3-cyano-5-methoxycarbonyl-N-arylpyrazoles as building blocks for the diversity-oriented synthesis of pyrazole-based polycyclic scaffolds†
Abstract
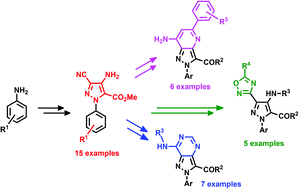
The synthesis of 4-amino-3-cyano-N-arylpyrazoles A based on a Thorpe–Ziegler cyclization as the key step has been achieved using microwave activation. Via a new diversity-oriented synthetic pathway, these highly functionalized building blocks allowed the access to various heteroaromatic scaffolds such as pyrazolo-pyridines B, pyrazolo-pyrimidines C and pyrazolo-oxadiazoles D. Interestingly, these platforms contain three to four reactive sites that could be used for post-functionalization in order to further increase the molecular diversity.

 Please wait while we load your content...
Please wait while we load your content...