Sc(OTf)3-mediated 1,3-dipolar cycloaddition–ring cleavage–rearrangement: a highly stereoselective access to Z-β-enaminonitriles†
Abstract
A novel and highly stereoselective synthesis of Z-β-enaminonitriles from azides and α,β-unsaturated nitriles is reported. The reaction proceeds via a 1,3-dipolar cycloaddition–ring cleavage–rearrangement cascade mediated by a catalytic amount of Sc(OTf)3. A plausible reaction mechanism for this process is depicted.
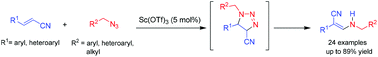

 Please wait while we load your content...
Please wait while we load your content...