E. coli cells expressing the Baeyer–Villiger monooxygenase ‘MO14’ (ro03437) from Rhodococcus jostii RHA1 catalyse the gram-scale resolution of a bicyclic ketone in a fermentor†
Abstract
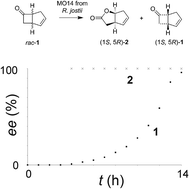
The Baeyer–Villiger monooxygenase (BVMO) ‘MO14’ from Rhodococcus jostii RHA1, is an enantioselective BVMO that catalyses the resolution of the model ketone substrate bicyclo[3.2.0]hept-2-en-6-one to the (1S,5R)-2-oxa lactone and the residual (1S,5R)-substrate enantiomer. This regio-plus enantioselective behaviour is highly unusual for BVMOs, which often perform enantiodivergent biotransformations of this substrate. The scaleability of the transformation was investigated using fermentor-based experiments, in which variables including gene codon optimisation, temperature and substrate concentration were investigated. E. coli cells expressing MO14 catalysed the resolution of bicyclo[3.2.0]hept-2-en-6-one to yield (1S,5R)-2-oxa lactone of >99% ee and (1S,5R)-ketone of 96% ee after 14 h at a temperature of 16 °C and a substrate concentration of 0.5 g L−1 (4.5 mM). MO14 is thus a promising biocatalyst for the production of enantio-enriched ketones and lactones derived from the [3.2.0] platform.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...