Copper-catalyzed regioselective synthesis of furan via tandem cycloaddition of ketone with an unsaturated carboxylic acid under air†
Abstract
A catalytic decarboxylative annulation has been developed for the regioselective synthesis of trisubstituted furans by the cycloaddition of ketones with α,β-unsaturated carboxylic acids under ambient air. A library of furan derivatives were obtained in good yields from the readily available substrates in the combination of a catalytic amount of Cu-salt and a stoichiometric amount of water. Water plays a crucial role in this catalytic transformation.
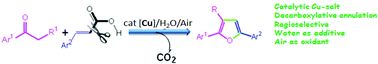

 Please wait while we load your content...
Please wait while we load your content...