Synthesis of sub-nanosized Pt particles on mesoporous SBA-15 material and its application to the CO oxidation reaction
Abstract
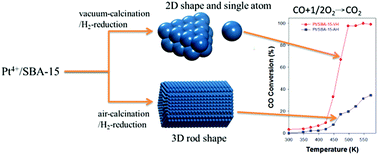
In this work, we show that the size and shape of Pt nanoparticles in SBA-15 can be controlled through vacuum and air calcination. The vacuum-calcination/H2-reduction process is used to thermally treat a 0.2 wt% Pt4+/SBA-15 sample to obtain small 2D clusters and single atoms that can significantly increase Pt dispersion in SBA-15. Compared with thermal treatments involving air-calcination/H2-reduction, which result in ∼4.6 nm rod-like Pt particles, vacuum-calcination/H2-reduction can dramatically reduce the size of the Pt species to approximately 0.5–0.8 nm. The Pt particles undergoing air-calcination/H2-reduction have poor conversion efficiency because the fraction of terrace sites, the major sites for CO oxidation, on the rod-like Pt particles is small. In contrast, a large amount of low-coordinated Pt sites associated with 2D Pt species and single Pt atoms in SBA-15 is effectively generated through the vacuum-calcination/H2-reduction process, which may facilitate CO adsorption and induce strong reactivity toward CO oxidation. We investigated the effect of vacuum-calcination/H2-reduction on the formation of tiny 2D clusters and single atoms by characterizing the particles, elucidating the mechanism of formation, determining the active sites for CO oxidation and measuring the heat of CO adsorption.

 Please wait while we load your content...
Please wait while we load your content...