Real-time, non-invasive monitoring of hydrogel degradation using LiYF4:Yb3+/Tm3+ NIR-to-NIR upconverting nanoparticles†
Abstract
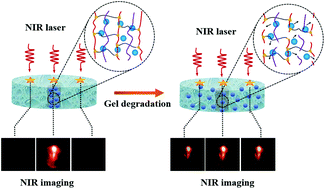
To design a biodegradable hydrogel as cell support, one should know its in vivo degradation rate. A technique commonly used to track gel degradation is fluorescence spectroscopy. However, the fluorescence from conventional fluorophores quickly decays, and the fluorophores are often moderately cytotoxic. Most importantly, they require ultraviolet or visible (UV-Vis) light as the excitation source, which cannot penetrate deeply through biological tissues. Lanthanide-doped upconverting nanoparticles (UCNPs) are exciting alternatives to conventional fluorophores because they can convert near-infrared (NIR) to UV-Vis-NIR light via a sequential multiphoton absorption process referred to as upconversion. NIR light can penetrate up to few cm inside tissues, thus making these UCNPs much better probes than conventional fluorophores for in vivo monitoring. Also, UCNPs have narrow emission bands, high photoluminescence (PL) signal-to-noise ratio, low cytotoxicity and good physical and chemical stability. Here, we show a nanocomposite system consisting of a biodegradable, in situ thermogelling injectable hydrogel made of chitosan and hyaluronic acid encapsulating silica-coated LiYF4:Yb3+/Tm3+ UCNPs. We use these UCNPs as photoluminescent tags to monitor the gel degradation inside live, cultured intervertebral discs (IVDs) over a period of 3 weeks. PL spectroscopy and NIR imaging show that NIR-to-NIR upconversion of LiYF4:Yb3+/Tm3+@SiO2 UCNPs allows for tracking of the gel degradation in living tissues. Both in vitro and ex vivo release of UCNPs follow a similar trend during the first 5 days; after this time, ex vivo release becomes faster than in vitro, indicating a faster gel degradation ex vivo. Also, the amount of released UCNPs in vitro increases continuously up to 3 weeks, while it plateaus after 15 days inside the IVDs showing a homogenous distribution of UCNPs throughout the IVD tissue. This non-invasive optical method for real time, live tissue imaging holds great potential for tissue analysis, biomapping and bioimaging applications.

 Please wait while we load your content...
Please wait while we load your content...