Metal–organic frameworks derived CoxFe1−xP nanocubes for electrochemical hydrogen evolution†
Abstract
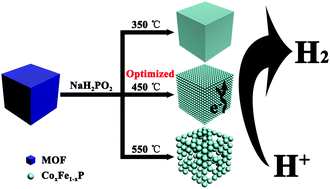
Designing and developing active, cost-effective and stable electrocatalysts for hydrogen evolution reaction (HER) are still an ongoing challenge. Herein, we report the synthesis of binary transition metal phosphide (CoxFe1−xP) nanocubes with different Co and Fe ratios through a phosphidation process using metal–organic frameworks (MOFs) as templates. MOF templates contribute well-defined nanocube architectural features after phosphidation, while a suitable phosphidation temperature could allow formation of a crystal structure and maintain the well-defined structure. The incorporation of a binary transition metal results in redistribution of the valence electrons in CoxFe1−xP. The changes imply anionic states of the P and Fe atoms, which act as active sites and thus have stronger electron-donating ability. When CoxFe1−xP nanocubes are employed as electrocatalysts, these characteristic features facilitate the performance of HER. Remarkably, Co0.59Fe0.41P nanocubes prepared at 450 °C afford a current density of 10 mA cm−2 at a low overpotential of 72 mV in acidic conditions and 92 mV in alkaline conditions. Co0.59Fe0.41P nanocubes also exhibit a small Tafel slope of 52 mV decade−1 in acidic conditions and 72 mV decade−1 in alkaline conditions. Moreover, Co0.59Fe0.41P nanocubes show good stability in both acidic and alkaline conditions. Our method produces the highly active HER catalyst based on binary transition metal MOF templates, providing a new avenue for designing excellent electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...