A novel form of β-strand assembly observed in Aβ33–42 adsorbed onto graphene†
Abstract
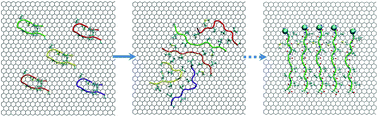
Peptide assembly plays a seminal role in the fabrication of structural and functional architectures in cells. Characteristically, peptide assemblies are often dominated by β-sheet structures, wherein component molecules are connected by backbone hydrogen bonds in a parallel or an antiparallel fashion. While β-rich peptide scaffolds are implicated in an array of neurodegenerative diseases, the mechanisms by which toxic peptides assemble and mediate neuropathic effects are still poorly understood. In this work, we employ molecular dynamics simulations to study the adsorption and assembly of the fragment Aβ33–42 (taken from the Aβ-42 peptide widely associated with Alzheimer's disease) on a graphene surface. We observe that such Aβ33–42 fragments, which are largely hydrophobic in character, readily adsorb onto the graphitic surface and coalesce into a well-structured, β-strand-like assembly. Strikingly, the structure of such complex is quite unique: hydrophobic side-chains extend over the graphene surface and interact with adjacent peptides, yielding a well-defined mosaic of hydrophobic interaction patches. This ordered structure is markedly depleted of backbone hydrogen bonds. Hence, our simulation results reveal a distinct type of β-strand assembly, maintained by hydrophobic side-chain interactions. Our finding suggests the backbone hydrogen bond is no longer crucial to the peptide assembly. Further studies concerning whether such β-strand assembly can be realized in other peptide systems and in biologically-relevant contexts are certainly warranted.

 Please wait while we load your content...
Please wait while we load your content...