Surface charge effects in protein adsorption on nanodiamonds†
Abstract
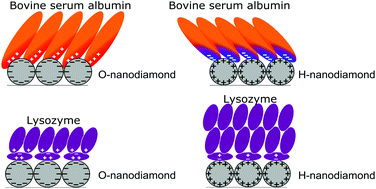
Understanding the interaction of proteins with charged diamond nanoparticles is of fundamental importance for diverse biomedical applications. Here we present a thorough study of protein binding, adsorption kinetics and structure on strongly positively (hydrogen-terminated) and negatively (oxygen-terminated) charged nanodiamond particles using a quartz crystal microbalance by dissipation and infrared spectroscopy. By using two model proteins (bovine serum albumin and lysozyme) of different properties (charge, molecular weight and rigidity), the main driving mechanism responsible for the protein binding to the charged nanoparticles was identified. Electrostatic interactions were found to dominate the protein adsorption dynamics, attachment and conformation. We developed a simple electrostatic model that can qualitatively explain the observed adsorption behaviour based on charge-induced pH modifications near the charged nanoparticle surfaces. Under neutral conditions, the local pH around the positively and negatively charged nanodiamonds becomes very high (11–12) and low (1–3) respectively, which has a profound impact on the protein charge, hydration and affinity to the nanodiamonds. Small proteins (lysozyme) were found to form multilayers with significant conformational changes to screen the surface charge, while larger proteins (albumin) formed monolayers with minor conformational changes. The findings of this study provide a step forward toward understanding and eventually predicting nanoparticle interactions with biofluids.


 Please wait while we load your content...
Please wait while we load your content...