Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction†
Abstract
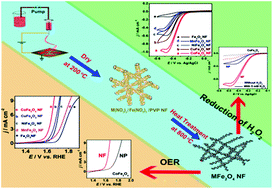
Designing and preparing porous transition metal ferrites without using any template, shape-directing agent, and surfactant is a challenge. Herein, heterojunction MFe2O4 (M = Co, Ni, Cu, Mn) nanofiber (NF) based films with three-dimensional configurations were synthesized by electrospinning and the subsequent thermal treatment processes. Characterization results indeed show the 3D net-like textural structures of the electrospun spinel-type MFe2O4 NFs. In particular, the resulting MFe2O4 NFs have lengths up to several dozens of micrometers with an average diameter size of about 150 nm and possess abundant micro/meso/macropores on both the surface and within the films. The hierarchically porous structures and high surface areas of these MFe2O4 NFs (for example, the CoFe2O4 NFs possess a larger BET specific surface area (61.48 m2 g−1) than those of the CoFe2O4 NPs (5.93 m2 g−1)) can afford accessible transport channels for effectively decreasing the mass transport resistances, enhancing the electrical conductivity, and increasing the density and reactivity of the exposed catalytic active sites. All these advantages will be responsible for the better electrocatalytic performances of these MFe2O4 NFs compared with their structural isomers (i.e. the MFe2O4 NPs) for the oxygen evolution reaction (OER) and H2O2 reduction in alkaline solution. Meanwhile, both the OER and H2O2 reduction catalytic activities for these MFe2O4 NFs obey the order of CoFe2O4 NFs > CuFe2O4 NFs > NiFe2O4 NFs > MnFe2O4 NFs > Fe2O3 NFs. The CoFe2O4 NFs represent a new class of highly efficient non-noble-metal catalysts for both OER and H2O2 reduction/detection in alkaline media.

 Please wait while we load your content...
Please wait while we load your content...