Copper nanoparticles of well-controlled size and shape: a new advance in synthesis and self-organization†
Abstract
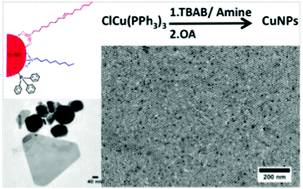
Here, we report a new synthetic route for spherical small copper nanoparticles (CuNPs) with size ranging from 3.5 nm to 11 nm and with an unprecedented associated monodispersity (<10%). This synthesis is based on the reduction of an organometallic precursor (CuCl(PPh3)3) by tert-butylamine borane in the presence of dodecylamine (DDA) at a moderate temperature (50 to 100 °C). Because of their narrow size distribution, the CuNPs form long-range 2D organizations (several μm2). The wide range of CuNPs sizes is obtained by controlling the reaction temperature and DDA-to-copper phosphine salt ratio during the synthesis process. The addition of oleic acid (OA) after the synthesis stabilizes the CuNPs (no coalescence) for several weeks under a nitrogen atmosphere. The nature and the reactivity of the ligands were studied by IR and UV-visible spectroscopy. We thus show that just after synthesis the nanoparticles are coated by phosphine and DDA. After adding OA, a clear exchange between phosphine and OA is evidenced. This exchange is possible thanks to an acid–base reaction between the free alkylamine in excess in the solution and OA. OA is then adsorbed on the NPs surface in the form of carboxylate. Furthermore, the use of oleylamine (OYA) instead of DDA as the capping agent allows one to obtain other NP shapes (nanorods, triangles and nanodisks). We get evidence that OYA allows the selective adsorption of chloride ions derived from the copper precursor on the different crystallographic faces during the growth of CuNPs that induces the formation of anisotropic shapes such nanorods or triangles.

 Please wait while we load your content...
Please wait while we load your content...