Photoluminescent carbon–nitrogen quantum dots as efficient electrocatalysts for oxygen reduction†
Abstract
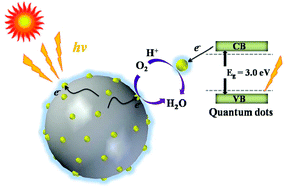
Here, we demonstrate novel carbon–nitrogen quantum dots (CNQDs) by the hydrothermal method using melamine and glutaraldehyde. CNQDs are uniformly dispersed with particle diameters of 3–8 nm. These prepared CNQDs show excellent luminescence properties and the photoluminescent quantum yields of CNQDs with 365 nm emission are up to 31%. Interestingly, CNQDs possess the co-existence of both p- and n-type conductivities from the Mott–Schottky relationship. Furthermore, the electrochemical measurements reveal that CNQDs exhibit catalytic activity for the oxygen reduction reaction (ORR). However, the low electrical conductivity affects the ORR electrocatalytic activity. So the catalysis is conducted by chemical coupling of CNQDs on Ag nanoparticles (NPs), the resulting CNQD/Ag NP catalyst is demonstrated to possess superior electrocatalytic ability for the ORR in an alkaline medium.

 Please wait while we load your content...
Please wait while we load your content...