A 2D–3D structure transition of gold clusters on CeO2−X(111) surfaces and its influence on CO and O2 adsorption: a comprehensive DFT + U investigation†
Abstract
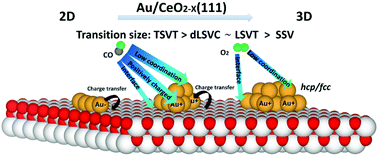
Detailed knowledge of the structures of gold nanoparticles on ceria surfaces is of fundamental importance to understand their extraordinary activities in catalysis. In this work, we employ density functional theory with the inclusion of the on-site Coulomb interaction (DFT + U) to investigate the structure evolution of small-sized gold (Au, Au4, Au8 and Au12) clusters on four types of reduced CeO2−X(111) surfaces: SSV (single surface oxygen vacancy), LSVT (linear surface oxygen vacancy trimer), dLSVC (double linear surface oxygen vacancy with a surface vacancy dimer and a subsurface vacancy), and TSVT (triangular surface oxygen vacancy trimer). Our results indicate that the relative stabilities of multilayer (3D) structures are strengthened gradually compared with the monolayer (2D) structures with increasing the number of gold atoms. In addition, the 2D–3D structure transition occurs on the size order of Au2D→3D@TSVT > Au2D→3D@dLSVC ∼ Au2D→3D@LSVT > Au2D→3D@SSV, which is determined by the charge transfer magnitude between the CeO2 surfaces and gold clusters. Meanwhile, two competitive nucleation patterns are observed, fcc-like nucleation and hcp-like nucleation, which highly affect the morphology of supported gold clusters. Further site-by-site investigations indicate that the coordination number and the charges of Au atoms are the dominant factors to influence the adsorption strength of CO and O2, and the interface plays a relatively minor role. These findings not only enrich our knowledge of the relationship between surface defects, gold cluster structures and small molecule adsorptions, but also provide a theoretical perspective to help design the optimal Au/CeO2 systems possessing high catalytic efficiency.


 Please wait while we load your content...
Please wait while we load your content...