Interrupted adenylation domains: unique bifunctional enzymes involved in nonribosomal peptide biosynthesis
Abstract
Covering up to 2014
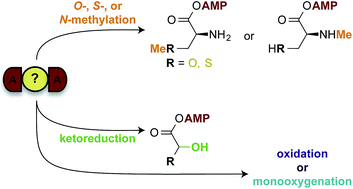
Nonribosomal peptides (NRPs) account for a large portion of drugs and drug leads currently available in the pharmaceutical industry. They are one of two main families of natural products biosynthesized on megaenzyme assembly-lines composed of multiple modules that are, in general, each comprised of three core domains and on occasion of accompanying auxiliary domains. The core adenylation (A) domains are known to delineate the identity of the specific chemical components to be incorporated into the growing NRPs. Previously believed to be inactive, A domains interrupted by auxiliary enzymes have recently been proven to be active and capable of performing two distinct chemical reactions. This highlight summarizes current knowledge on A domains and presents the various interrupted A domains found in a number of nonribosomal peptide synthetase (NRPS) assembly-lines, their predicted or proven dual functions, and their potential for manipulation and engineering for chemoenzymatic synthesis of new pharmaceutical agents with increased potency.
- This article is part of the themed collection: Biosynthetic Assembly Lines

 Please wait while we load your content...
Please wait while we load your content...