Synthesis and structural characterization of Zn-containing DAF-1†
Abstract
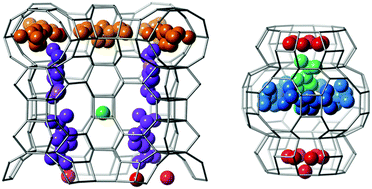
A study exploring the use of ionic liquid reactions based on imidazolium halides in molecular sieve synthesis has produced a novel zincoaluminophosphate material with an open DFO-type framework structure. This framework structure had only been observed previously in the magnesioaluminophosphate system (Mg-DAF-1) where decamethonium was used as the structure directing agent. The new Zn-DAF-1 material has been characterized using chemical and thermogravimetric analysis and 13C, 19F, 27Al and 31P MAS NMR techniques. Structure analysis (P6/mcc, a = 22.2244(1) Å, c = 42.3293(3) Å) using synchrotron powder diffraction data not only confirmed the framework structure, but also revealed the locations of the Al, P and Zn atoms in the framework, the N,N′-di-isopropyl-imidazolium (DIPI) ions in the pores, some fluoride ions associated with double 4-rings, and some water molecules and anions filling the remaining space. This level of structural detail had not been possible in the Mg-DAF-1 material. Four different locations for the DIPI cation were found in the two 12-ring channels and Zn was found to substitute for only one of the six crystallographically distinct Al sites to yield the approximate crystal chemical formula |(DIPI)17(OH,F)11(H2O)23|[Zn6Al126P132O528]-DFO.
- This article is part of the themed collection: The Creative World of Porous Materials


 Please wait while we load your content...
Please wait while we load your content...