The excited-state multiple proton transfer mechanism of the 7-hydroxyquinoline–(CH3OH)3 cluster†
Abstract
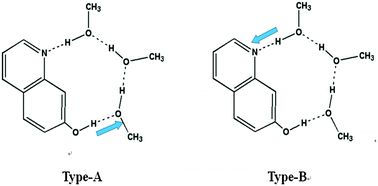
In this paper, the excited-state proton transfer (ESPT) of 7-hydroxyquinoline (7HQ) in hydrogen-donating methanol (MeOH) was investigated theoretically. The change in the calculated primary bond lengths and the infrared spectra of the 7HQ–(CH3OH)3 configuration from the S0 state to the S1 state testify that the intermolecular hydrogen bonds were strengthened in the S1 state. Therefore, we propose two proton transfer (PT) types in which the ESPT reaction occurs along the hydrogen-bonded bridge O7⋯H8–O9 (Type-A) or N1⋯H2–O3 (Type-B). The normal Stokes shift and the large Stokes shift suggest the existence of the enol form 7HQ–(CH3OH)3 and the keto form 7HQ–(CH3OH)3-PT, respectively, and thus that the proton transfer can occur in the excited-state. In order to further study the details of the complicated multiple proton transfer of the 7HQ–(CH3OH)3 cluster, we scanned the potential energy curves for the ground state (S0) and the excited state (S1) of the hydrogen-bonded 7HQ–(CH3OH)3 complex along with the hydrogen-bonding coordinate (distance of the H2–O3 and H8–O9 bonds). The results show the close barrier height between Type-A (5.35 kcal mol−1) and Type-B (6.65 kcal mol−1), which indicates that the two possible PT types may co-exist and have competition between themselves.

 Please wait while we load your content...
Please wait while we load your content...