Synthetic bacteriochlorins bearing polar motifs (carboxylate, phosphonate, ammonium and a short PEG). Water-solubilization, bioconjugation, and photophysical properties†
Abstract
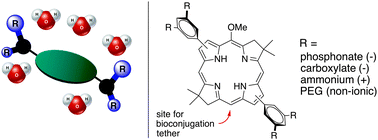
Bacteriochlorins are potentially excellent chromophores for near-infrared (NIR) photochemical and spectroscopic studies yet the intrinsically hydrophobic macrocycle core has stymied work in aqueous media. Herein, a set of bacteriochlorins bearing distinct polar motifs is reported. The motifs include phosphonate (pH-dependent anionic, BC1), carboxylate (pH-dependent anionic, BC2), ammonium (permanently cationic) without (BC3) or with (BC4) a linker ester moiety, and tetraethyleneoxy (a short PEG, polar non-ionic, BC5). The groups are located at the 3,5-positions of each of two aryl groups at the bacteriochlorin 3,13-sites. Synthesis of the bacteriochlorins entails the Suzuki coupling of a common 3,13-dibromobacteriochlorin building block with a set of aryl boronates. Five factors were selected for comparisons among the polar motifs upon attachment to the bacteriochlorins: (1) synthesis yield and ease of purification, (2) amenability toward subsequent derivatization, (3) water-solubility, (4) full-width-at-half-maximum (fwhm) of the long-wavelength (Qy) absorption and fluorescence bands, singlet excited-state lifetime (τS) and fluorescence quantum yield (Φf), and (5) stability in the dark or under illumination. Water-solubility was assessed by examination of the absorption spectra across a 1000-fold concentration range (∼0.2–0.6 μM to ∼200–600 μM). With the exception of BC4, all displayed good aqueous solubility, photostability, and photophysical properties in aqueous solution (fwhm = 23–31 nm, Φf = 0.10–0.16, τS = 1.9–2.7 ns). The modestly lower Φf and τS values for the bacteriochlorins in aqueous versus organic (N,N-dimethylformamide) media are traced to an increased rate constant for excited-state internal conversion. Upon consideration of all factors, the ammonium (short linker) and short PEG groups were most attractive for solubilization of the bacteriochlorins in aqueous media. The studies prompted the synthesis of two water-soluble (ammonium-substituted) bacteriochlorins bearing N-hydroxysuccinimide esters.

 Please wait while we load your content...
Please wait while we load your content...