Abstract
Reactions of anhydrous lanthanide trichlorides, LnCl3 (Ln = Nd, Sm, Ho), with 3 equiv. of lithium-cyclopropylethinylamidinates, Li[c-C3H5–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(NR)2] (1a: R = cyclohexyl (Cy), 1b: R = iPr), afforded the new homoleptic lanthanide(III) tris(cyclopropylethinylamidinate) complexes [c-C3H5–C
C–C(NR)2] (1a: R = cyclohexyl (Cy), 1b: R = iPr), afforded the new homoleptic lanthanide(III) tris(cyclopropylethinylamidinate) complexes [c-C3H5–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(NCy)2]3Sm (2a) and [c-C3H5–C
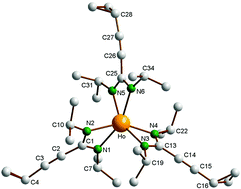
C–C(NCy)2]3Sm (2a) and [c-C3H5–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(NiPr)2]3Ln (Ln = Nd (2b), Sm (2c), Ho (2d)) as air- and moisture-sensitive crystalline solids in moderate to good isolated yields (45–79%). The formation of unsolvated, homoleptic Ln(III) tris(cyclopropylethinylamidinate) was confirmed by an X-ray diffraction study of the holmium derivative [c-C3H5–C
C–C(NiPr)2]3Ln (Ln = Nd (2b), Sm (2c), Ho (2d)) as air- and moisture-sensitive crystalline solids in moderate to good isolated yields (45–79%). The formation of unsolvated, homoleptic Ln(III) tris(cyclopropylethinylamidinate) was confirmed by an X-ray diffraction study of the holmium derivative [c-C3H5–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(NiPr)2]3Ho (2d). EI mass spectra of the new rare-earth metal amidinates indicated a significant volatility. An initial catalysis study revealed that these complexes catalyze the addition of terminal alkynes to carbodiimides to give propiolamidines of the type R–C
C–C(NiPr)2]3Ho (2d). EI mass spectra of the new rare-earth metal amidinates indicated a significant volatility. An initial catalysis study revealed that these complexes catalyze the addition of terminal alkynes to carbodiimides to give propiolamidines of the type R–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(
C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR′)(NHR′). The molecular structure of N,N′-dicyclohexyl-phenylpropiolamidine, Ph–C
NR′)(NHR′). The molecular structure of N,N′-dicyclohexyl-phenylpropiolamidine, Ph–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(NCy)(NHCy) (4), was also determined by X-ray diffraction.
C–C(NCy)(NHCy) (4), was also determined by X-ray diffraction.
- This article is part of the themed collection: Frontiers of Organo-f-Element Chemistry

 Please wait while we load your content...
Please wait while we load your content...