Exploring the halogen bond specific solvent effects in halogenated solvent systems by ESR probe†
Abstract
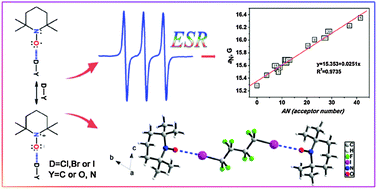
The halogen bond specific solvent effect in halogenated solvents was investigated by ESR, Raman spectroscopy, X-ray diffraction and unrestricted MP2 calculations using the ESR probe TEMPO. From ESR spectroscopy, 14N isotropic hyperfine splitting (HFS) constant aN values are higher in halogenated than non-halogenated solvents as solvent polarity is near to each other, and plotting aN values vs. solvent acceptor number (AN) provides a linear correlation. These results could be attributed to the formation of halogen bonding (XB) and indicate that as specific solvent effect halogen bonding does exist in halogenated solvents, particularly in halo-perfluoro-solvents, in which the halogen bonding between the nucleophilic solutes and halogenated solvent molecules is so strong that it must not be ignored. In both halogen- and hydrogen-containing solvents, halogen bonding and hydrogen bonding may co-exist. Calculations show that the strength of hydrogen bond may be slightly stronger than the corresponding halogen bond. However, the halogen bond becomes more competitive going from chlorine(Cl)-substituted to bromine(Br)-substituted to iodine(I)-substituted solvents, and it is possible that XB solvent effects become preponderant in I-substituted solvents. The analysis of Mulliken atomic spin densities on N and O atoms also proved that when the XB is formed, the spin densities on O would transfer to N, further indicating that the increase of aN should be ascribed to XB specific solvent effects.

 Please wait while we load your content...
Please wait while we load your content...