A one-pot ‘click’ reaction from spiro-epoxides catalyzed by Cu(i)-pyrrolidinyl-oxazole-carboxamide†
Abstract
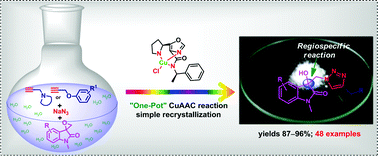
An efficient Cu(I)-pyrrolidinyl-oxazolo-carboxamide catalyst has been employed for the ‘one-pot’ synthesis of novel 3-substituted-1,2,3-triazolo-3-hydroxy-indolin-2-ones. This reaction involves an in situ azide generation from spiro-epoxide by a concomitant ‘click’ reaction in aqueous media. This protocol has the advantage of avoiding the interim purification of toxic organic azide intermediates resulting in significant enhancement of the overall yield with reduced reaction time. The regiospecificity of epoxide ring-opening has been unequivocally established on the basis of single X-ray crystallographic analysis and quantum chemical calculations. Moreover, this approach offers a broad scope to access diversely substituted indolino-O/N-linked 1,2,3-triazoles as novel privileged scaffolds.


 Please wait while we load your content...
Please wait while we load your content...