The influence of zinc(ii) on thioredoxin/glutathione disulfide exchange: QM/MM studies to explore how zinc(ii) accelerates exchange in higher dielectric environments†
Abstract
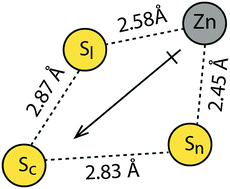
QM/MM studies were performed to explore the energetics of exchange reactions of glutathione disulfide (GSSG) and the active site of thioredoxin [Cys32–Gly33–Pro34–Cys35] with and without zinc(II), in vacuum and solvated models. The activation energy for exchange, in the absence of zinc, is 29.7 kcal mol−1 for the solvated model. This is 3.3 kcal mol−1 higher than the activation energy for exchange in the gas phase, due to ground state stabilization of the active site Cys-32 thiolate in a polar environment. In the presence of zinc, the activation energy for exchange is 4.9 kcal mol−1 lower than in the absence of zinc (solvated models). The decrease in activation energy is attributed to stabilization of the charge-separated transition state, which has a 4-centered, cyclic arrangement of Zn–S–S–S with an estimated dipole moment of 4.2 D. A difference of 4.9 kcal mol−1 in activation energy would translate to an increase in rate by a factor of about 4000 for zinc-assisted thiol-disulfide exchange. The calculations are consistent with previously reported experimental results, which indicate that metal-thiolate, disulfide exchange rates increase as a function of solvent dielectric. This trend is opposite to that observed for the influence of the dielectric environment on the rate of thiol-disulfide exchange in the absence of metal. The results suggest a dynamic role for zinc in thiol-disulfide exchange reactions, involving accessible cysteine sites on proteins, which may contribute to redox regulation and mechanistic pathways during oxidative stress.
- This article is part of the themed collection: Zinc in the Biosciences

 Please wait while we load your content...
Please wait while we load your content...