Dynamic HypA zinc site is essential for acid viability and proper urease maturation in Helicobacter pylori
Abstract
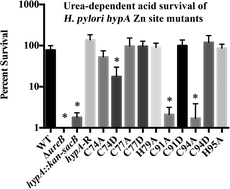
Helicobacter pylori requires urease activity in order to survive in the acid environment of the human stomach. Urease is regulated in part by nickelation, a process that requires the HypA protein, which is a putative nickel metallochaperone that is generally associated with hydrogenase maturation. However, in H. pylori, HypA plays a dual role. In addition to an N-terminal nickel binding site, HypA proteins also contain a structural zinc site that is coordinated by two rigorously conserved CXXC sequences, which in H. pylori are flanked by His residues. These structural Zn sites are known to be dynamic, converting from Zn(Cys)4 centers at pH 7.2 to Zn(Cys)2(His)2 centers at pH 6.3 in the presence of Ni(II) ions. In this study, mutant strains of H. pylori that express zinc site variants of the HypA protein are used to show that the structural changes in the zinc site are important for the acid viability of the bacterium, and that a reduction in acid viability in these variants can be traced in large measure to deficient urease activity. This in turn leads to a model that connects the Zn(Cys)4 coordination to urease maturation.
- This article is part of the themed collections: Zinc in the Biosciences and Nickel in biology

 Please wait while we load your content...
Please wait while we load your content...