Novel insights into nickel import in Staphylococcus aureus: the positive role of free histidine and structural characterization of a new thiazolidine-type nickel chelator†
Abstract
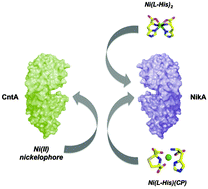
Staphylococcus aureus possesses two canonical ABC-importers dedicated to nickel acquisition: the NikABCDE and the CntABCDF systems, active under different growth conditions. This study reports on the extracytoplasmic nickel-binding components SaNikA and SaCntA. We showed by protein crystallography that SaNikA is able to bind either a Ni-(L-His)2 complex or a Ni-(L-His) (2-methyl-thiazolidine dicarboxylate) complex, depending on their availability in culture supernatants. Native mass spectrometry experiments on SaCntA revealed that it binds the Ni(II) ion via a different histidine-dependent chelator but it cannot bind Ni-(L-His)2. In vitro experiments are consistent with in vivo nickel content measurements that showed that L-histidine has a high positive impact on nickel import via the Cnt system. These results suggest that although both systems may require free histidine, they use different strategies to import nickel.
- This article is part of the themed collection: Nickel in biology

 Please wait while we load your content...
Please wait while we load your content...