Design and discovery of 3-aryl-5-substituted-isoquinolin-1-ones as potent tankyrase inhibitors†
Abstract
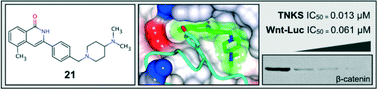
The tankyrase proteins (TNKS, TNKS2), members of the PARP superfamily of enzymes, are attractive anti-cancer drug targets, particularly as inhibition of their catalytic activity has been shown to antagonise oncogenic WNT signalling. To identify chemical inhibitors of tankyrase we carried out an in silico small molecule screen using a set of ‘PARP-binding’ pharmacophores together with a generated (liganded) tankyrase homology model. This approach identified a structurally diverse set of ~1000 compounds for further study. Subsequent in vitro screening of recombinant tankyrase protein identified a subset of 59 confirmed inhibitors. Early optimisation followed by cell-based studies in WNT-dependent tumour cells, as well as co-crystallisation studies, identified a novel class of 3-aryl-5-substituted isoquinolin-1-ones, such as 21, that exhibit potent inhibition of tankyrase activity as well as growth inhibition of colorectal cancer cells.

 Please wait while we load your content...
Please wait while we load your content...