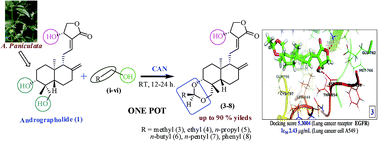
A series of 3,19-O-acetal derivatives of andrographolide (1) have been synthesized by protecting the hydroxyls at C-3 and C-19 through a novel route. All the derivatives were evaluated for in vitro anticancer activity. Among the synthesized derivatives, compounds 3, 3a, 3d, 3e, 7 and 8 showed potential cytotoxicity against human cancer cell lines A549 (lung), HeLa (cervical), ACHN (renal), B-16 (melanoma) and IEC-6 (small intestine). The binding mode conformation was evaluated through docking simulations, while bioavailability/drug-likeness was evaluated through predictive ADME screening studies. All the derivatives were characterized by spectroscopy and the stereochemistry of compounds 3a and 3e was also confirmed by X-ray analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...