Autoantigenicity of human C1q is associated with increased hydrophobicity due to conformational transitions in the globular heads
Abstract
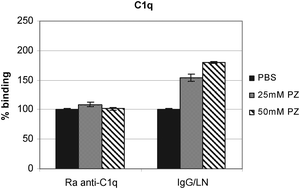
We analyzed the structural features of C1q that underlie its autoantigenicity by means of a model system using the amphiphilic polyzwitterion (PZ), poly(ethylene oxide-b-N,N-dimethyl(methacryloyloxyethyl) ammonium propanesulfonate) in the process of C1q immobilization. The source of anti-C1q autoantibodies was human sera from patients with Lupus Nephritis (LN). Both analyzed concentrations of PZ, 25 mM and 50 mM, were found to be applicable for inducing conformational transitions which resulted in increased recognition of C1q and the globular domain of its B polypeptide chain, designated ghB, by the LN autoantibodies. The registered conformational transitions displayed a hydrophobic enhancement of the protein microenvironment due to the presence of hydrophobic binding sites in ghB which consequently affected the autoantigenicity of the whole C1q molecule.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...