Human 3-hydroxyanthranilate 3,4-dioxygenase (3HAO) dynamics and reaction, a multilevel computational study†
Abstract
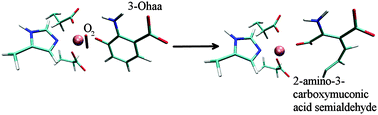
3-Hydroxyanthranilate 3,4-dioxygenase (3HAO) is a non-heme iron dependent enzyme. It catalyses the cleavage of the benzene ring of 3-hydroxyanthranilic acid (3-Ohaa), an intermediate in the kynurenine pathway, and therefore represents a potential target in treating numerous disorders related to the concentration of quinolinic acid (QUIN), the kynurenine pathway product, in tissues. The stability and behaviour of the enzyme in nearly physiological conditions, studied by the empirical molecular modelling methods enabled us to determine the influence of several, for the enzyme activity relevant, point mutations (Arg43Ala, Arg95Ala and Glu105Ala) on the protein structure, particularly on the active site architecture and the metal ion environment, as well as on the substrate, 3-Ohaa, binding. Besides, the water population of the active site, and the protein flexibility as well as the amino acid residues interaction networks relevant for the enzyme activity were determined for the 3-Ohaa complexes with the native and mutated enzyme variants. Finally, using the hybrid quantum-mechanics/molecular-mechanics (QM/MM) calculations the 3HAO catalysed 3-Ohaa oxidation into 2-amino-3-carboxymuconic acid semialdehyde was elucidated.


 Please wait while we load your content...
Please wait while we load your content...