Combining bio- and chemo-catalysis for the conversion of bio-renewable alcohols: homogeneous iridium catalysed hydrogen transfer initiated dehydration of 1,3-propanediol to aldehydes†
Abstract
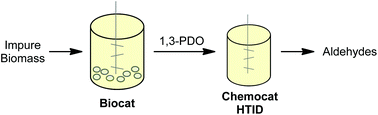
Combining whole cell biocatalysis and chemocatalysis in a single reaction sequence avoids unnecessary separations, and the associated waste and energy consumption. Bacterial fermentation has been employed to convert waste glycerol from biodiesel production into 1,3-propanediol. This 1,3-propanediol can be extracted selectively from the aqueous fermentation broth using ionic liquids. 1,3-Propanediol in ionic liquid solution was converted to propionaldehyde by hydrogen transfer initiated dehydration (HTID) catalysed by a Cp*IrCl2(NHC) (Cp* = pentamethylcyclopentadienyl; NHC = carbene ligand) complex. The use of an ionic liquid solvent enabled the reaction to be performed under reduced pressure, facilitating the isolation of the product, and improving the reaction selectivity. The Ir(III) catalyst in ionic liquid was found to be highly recyclable.
- This article is part of the themed collection: 3rd International Symposium on Green Chemistry

 Please wait while we load your content...
Please wait while we load your content...