Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles†
Abstract
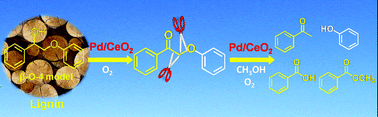
The oxidative transformation of lignin into aromatic compounds is an attractive route for chemical utilization of lignocellulosic biomass. Unlike hydrogenolysis, no consumption of expensive hydrogen is required for the oxidative transformation. However, only limited success has been achieved for the oxidative conversion of lignin. Here, we report that cerium oxide-supported palladium nanoparticles (Pd/CeO2) can efficiently catalyze the one-pot oxidative conversion of 2-phenoxy-1-phenylethanol, a lignin model compound containing a β-O-4 bond and a Cα-hydroxyl group, in methanol in the presence of O2, producing phenol, acetophenone and methyl benzoate as the major products. Pd nanoparticles played a pivotal role in the oxidation of a Cα-hydroxyl group into a Cα-ketonic group, which was crucial for the transformation of the model compound. The presence of the Cα-ketonic group activated the β-O-4 bond, which was subsequently cleaved over the Pd/CeO2 catalyst, affording phenol and acetophenone. At the same time, the Cα–Cβ bond also underwent oxidative cleavage catalyzed by CeO2, producing benzoic acid and further methyl benzoate. The Pd/CeO2 catalyst could also catalyze the oxidative conversion of organosolv lignin under mild conditions (458 K), producing vanillin, guaiacol and 4-hydroxybenzaldehyde.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...