Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics†
Abstract
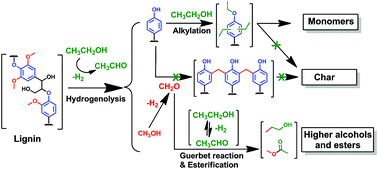
Obtaining renewable fuels and chemicals from lignin presents an important challenge to the use of lignocellulosic biomass to meet sustainability and energy goals. We report on a thermocatalytic process for the depolymerization of lignin in supercritical ethanol over a CuMgAlOx catalyst. Ethanol as solvent results in much higher monomer yields than methanol. In contrast to methanol, ethanol acts as a scavenger of formaldehyde derived from lignin decomposition. Studies with phenol and alkylated phenols evidence the critical role of the phenolic –OH groups and formaldehyde in undesired repolymerization reactions. O-alkylation and C-alkylation capping reactions with ethanol hinder repolymerization of the phenolic monomers formed during lignin disassembly. After reaction in ethanol at 380 °C for 8 h, this process delivers high yields of mainly alkylated mono-aromatics (60–86 wt%, depending on the lignin used) with a significant degree of deoxygenation. The oxygen-free aromatics can be used to replace reformate or can serve as base aromatic chemicals; the oxygenated aromatics can be used as low-sooting diesel fuel additives and as building blocks for polymers.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...