Direct asymmetric reduction of levulinic acid to gamma-valerolactone: synthesis of a chiral platform molecule†
Abstract
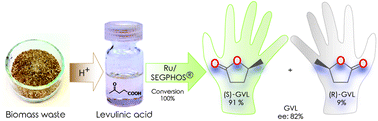
Levulinic acid was directly converted to optically active (S)-gamma-valerolactone, a proposed biomass-based chiral platform molecule. By using a SEGPHOS ligand-modified ruthenium catalyst in methanol as a co-solvent, eventually, 100% chemoselectivity, and 82% enantioselectivity were achieved. The effect of the catalyst composition and reaction parameters on the activity and selectivity was investigated in detail. The conversion of a “real” biomass derived levulinic acid to optically active GVL without decreasing the enantioselectivity was also demonstrated.
- This article is part of the themed collection: Green solvents for synthesis

 Please wait while we load your content...
Please wait while we load your content...