Deep eutectic solvents: biorenewable reaction media for Au(i)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels–Alder reactions†
Abstract
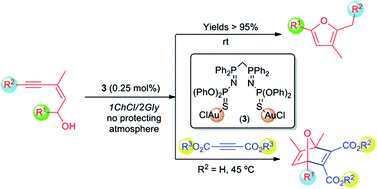
Cycloisomerisation reactions of (Z)-enynols into furans can be conveniently performed, for the first time, in the eutectic mixture 1ChCl/2Gly as a solvent and under standard bench conditions (at room temperature and under air) by using the new bis(iminophosphorane)–Au(I) complex [Au2Cl2(μ2-S,S-CH2{P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NP(
NP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)(OPh)2)Ph2}2)] as a catalyst. Furthermore, a one-pot tandem reaction involving the fast cycloisomerisation of (Z)-enynols followed by an intermolecular atom economical process, i.e. the Diels–Alder reaction with activated alkynes or alkenes, is reported. This tandem cycloisomerisation/Diels–Alder reaction proceeds also in the eutectic mixture 1ChCl/2Gly, in the absence of co-catalysts and under aerobic conditions, giving rise to the corresponding 7-oxanorbornadienes and 7-oxanorbornenes under the principles of the so-called Green Chemistry.
S)(OPh)2)Ph2}2)] as a catalyst. Furthermore, a one-pot tandem reaction involving the fast cycloisomerisation of (Z)-enynols followed by an intermolecular atom economical process, i.e. the Diels–Alder reaction with activated alkynes or alkenes, is reported. This tandem cycloisomerisation/Diels–Alder reaction proceeds also in the eutectic mixture 1ChCl/2Gly, in the absence of co-catalysts and under aerobic conditions, giving rise to the corresponding 7-oxanorbornadienes and 7-oxanorbornenes under the principles of the so-called Green Chemistry.

 Please wait while we load your content...
Please wait while we load your content...