Lignin repolymerisation in spruce autohydrolysis pretreatment increases cellulase deactivation†
Abstract
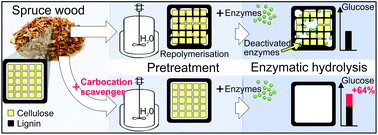
This study presents a modified autohydrolysis pretreatment which helps to overcome the recalcitrance of softwood for enzymatic hydrolysis of its cellulose. Autohydrolysis pretreatments of spruce wood were performed with 2-naphthol, which prevents lignin repolymerisation reactions, thereby increasing the enzymatic digestibility of cellulose by up to 64%. The negative influence of repolymerised lignin structures on enzymatic hydrolysis was confirmed by the addition of resorcinol in autohydrolysis, which is known to promote repolymerisation reactions and decreased the biomass digestibility. Several analyses were performed to study the underlying mechanism of this effect on hydrolysis, indicating that cellulolytic enzymes are adsorbed and deactivated especially by repolymerised lignin structures, which accounts for the high differences in biomass digestibility. It was shown that lignin repolymerisation significantly increases its specific surface area through modification of the lignin nanostructure, which is supposed to increase the unproductive binding of enzymes.

 Please wait while we load your content...
Please wait while we load your content...