A route to convert CO2: synthesis of 3,4,5-trisubstituted oxazolones†
Abstract
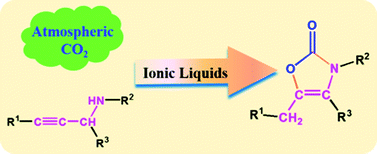
Production of value-added chemicals using carbon dioxide (CO2) as a feedstock is favorable to the sustainable development of the chemical industry. In this work, we have discovered for the first time that CO2 can react with propargylic amines to produce 3,4,5-trisubstituted oxazolones, a class of very useful chemicals. It was found that the ionic liquid (IL) 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) can catalyze the reactions efficiently at atmospheric pressure under metal-free conditions. It was also found that [Bmim][OAc] and IL 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([Bmim][Tf2N]) have an excellent synergistic effect for promoting the reactions. The [Bmim][OAc]/[Bmim][Tf2N] catalytic system can be reused at least five times without loss in catalytic activity and selectivity. The reaction mechanism was proposed on the basis of density functional theory (DFT) calculation and the experimental results.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...