Silver tungstate: a single-component bifunctional catalyst for carboxylation of terminal alkynes with CO2 in ambient conditions†
Abstract
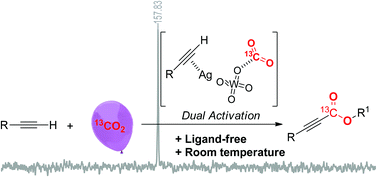
Silver tungstate was successfully developed as a bifunctional catalyst for the ligand-free carboxylation of various terminal alkynes with electron-withdrawing or electron-donating groups under atmospheric pressure of carbon dioxide (CO2) at room temperature. In this protocol, dual activation – i.e., the terminal alkyne activated by silver, and CO2 activation by the tungstate anion – was verified using nuclear magnetic resonance spectroscopy, and means that this reaction can be run under ambient conditions. Notably, this protocol can be applied to the preparation of a phenylacrylate derivative by a cascade reaction using phenylacetylene, CO2 and benzylamine as starting materials.

 Please wait while we load your content...
Please wait while we load your content...