A novel d-glucosamine-derived pyridyl-triazole@palladium catalyst for solvent-free Mizoroki–Heck reactions and its application in the synthesis of Axitinib†
Abstract
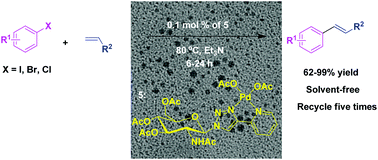
A green method for the synthesis of a D-glucosamine-derived triazole@palladium catalyst is described. The synthesized catalyst containing a 2-pyridyl-1,2,3-triazole ligand was prepared via a click route in high yields and was explored in Heck cross-coupling reactions between different aryl halides and olefins under solvent-free conditions. The catalyst can be separated from the reaction mixture and reused at least six times with superior activity. In addition, using this protocol, the marketed drug Axitinib (antitumor) could be synthesized easily.

 Please wait while we load your content...
Please wait while we load your content...