Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production†
Abstract
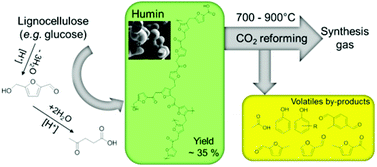
Lignocellulosic biomass is addressed as potential sustainable feedstock for green fuels and chemicals. (Hemi)cellulose is the largest constituent of the material. Conversion of these polysaccharides to bio-based platform chemicals is important in green chemical/fuel production and biorefinery. Hydroxymethyl furfural, furfural and levulinic acid are substantial building blocks from (poly)saccharides. Synthesis of these molecules involves acid catalysed hydrolysis/dehydration reactions which leads large formation of insoluble by-products, called humins. Humin obtained from dehydration of glucose is used in this study. Fractionisation of humin was investigated using various solvents (e.g., acetone, H2O, and NaOH 1%). Characterisation of humin using various techniques including ATR-IR, HR-SEM, solid state NMR, elemental analysis, Raman spectroscopy, pyrolysis, etc. confirms its furan rich structure with aliphatic oxygenate linkages. The influence of thermal treatment on humin was investigated. Humin undergoes a lot of changes both in morphology and structure. Humin loses ca. 45 wt% when preheated to 700 °C (prior to the gasification temperature) and contains above 92 wt% C in mainly aromatic/graphitic structures. Valorisation of humin via dry reforming was studied. Non-catalytic dry reforming of humin is very difficult; however, alkali catalysts (e.g. Na2CO3) can enhance the reaction rate tremendously.

 Please wait while we load your content...
Please wait while we load your content...