Phosphorus recovery from urine and anaerobic digester filtrate: comparison of adsorption–precipitation with direct precipitation†
Abstract
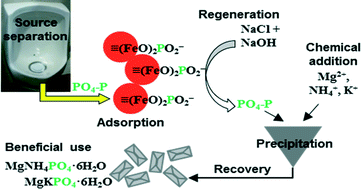
Hybrid anion exchange resin containing hydrous ferric oxide (HAIX-Fe) was used in column tests to remove phosphate (PO4) from fresh urine, hydrolyzed urine, and anaerobic digester filtrate, and subsequently recover PO4 as struvite (MgNH4PO4·6H2O) or potassium struvite (MgKPO4·6H2O) via precipitation in the spent regenerant. The recovery of PO4 using the two-step adsorption–precipitation process was compared with direct precipitation in urine and anaerobic digester filtrate considering chemical requirements for precipitation and mineral purity. Following the saturation of HAIX-Fe resin with PO4 from urine and anaerobic digester filtrate, up to 95% of the PO4 was desorbed using caustic brine during the regeneration phase. The spent regenerants were more concentrated in PO4 than the original urine and anaerobic digester filtrate due to recycling of the regeneration solution. Precipitation in the spent regenerants and original wastewaters (urine and filtrate) yielded 96.7–99.8% PO4 recovery as struvite or potassium struvite. Direct precipitation in fresh urine and hydrolyzed urine was more efficient than precipitation in the corresponding spent regenerants based on lower chemical requirements. Precipitation in the spent regenerant from HAIX-Fe resin saturated with PO4 from anaerobic digester filtrate produced a higher purity mineral than direct precipitation in the anaerobic digester filtrate.


 Please wait while we load your content...
Please wait while we load your content...