Heteroaggregation of bare silver nanoparticles with clay minerals†
Abstract
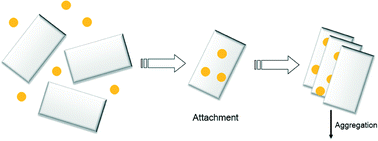
In this study, we investigated the heteroaggregation of silver nanoparticles with clay minerals in neutral pH solutions as a function of electrolyte type and concentration. Bare nanoparticles with a nominal diameter of 60.9 ± 0.5 nm were synthesized and their aggregation behavior with illite (IMt-2) and montmorillonite (SWy-2), pretreated to obtain monocationic (Na-clay) and dicationic (Ca-clay) suspensions, was studied. Aggregation was monitored as a function of electrolyte concentration in both homo- and heteroaggregation scenarios by measuring the change in hydrodynamic diameter as a function of time using dynamic light scattering. Our results did not show significant differences in the stability of binary component systems of bare silver nanoparticle and clays at pH 7 when compared to the single particle systems of clay or silver at the same pH. In each case, high electrolyte concentrations were needed to overcome the energy barrier to form aggregates, which we attribute to weakly charged or negatively charged clay edges, negatively charged silver nanoparticles, as well as the permanently negatively charged basal plane surfaces of the clays at pH 7. The results suggest that a binary system of clay (either montmorillonite or illite) and bare silver nanoparticles can be treated as a single component system of the respective clay under the experimental conditions studied, and the fate of silver nanoparticles in aqueous system may be controlled by their heteroaggregation with native particles, such as clays.

 Please wait while we load your content...
Please wait while we load your content...