Engineered manganese oxide nanocrystals for enhanced uranyl sorption and separation†
Abstract
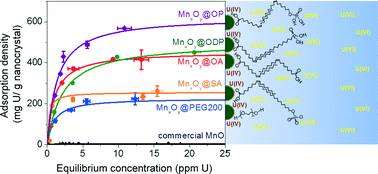
For the first time, this work develops and demonstrates precisely engineered manganese oxide nanoscale particles for the sorption of uranium, as uranyl, in water. Size controlled monodisperse nanocrystalline manganese oxides (12 to 28 nm) were systematically synthesized via thermal decomposition of manganese oleate and phase-transferred into water by ligand exchange and bilayer stabilization methods. Resulting monodisperse suspensions demonstrate significantly enhanced uranyl adsorption as a function of size, surface coating chemistries, and solution pH. In particular, 12 nm particles coated with the unsaturated–unsaturated carbon chains linked bilayers, (e.g. oleic acid-oleyl phosphate linked bilayer coatings) have binding capacities well over 600 mg U per g of Mn, which is the highest reported uranium sorption capacity for any manganese based sorbent to date. Further, we spectrally identify significant uranyl reduction as part of the adsorption mechanism(s) for high capacity materials. Last, oleyl-based (phosphate and carboxylic) functionalized bilayered nanocrystals were extremely stable in the presence of high ionic strength/type (>800 mM); calcium (>19 mM), including the presence of uranyl cations (from 0.1 to 60 ppm). Taken together, these data demonstrate the potential for engineered monodisperse manganese oxide nanocrystals as ultra-high capacity platform sorbent materials for uranium separation at environmentally relevant ionic strengths and pH.
- This article is part of the themed collection: Sustainable Nanotechnology Organization 2014

 Please wait while we load your content...
Please wait while we load your content...